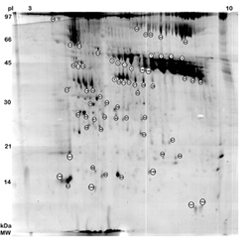
Food quality is not only a function of nutritional values but also of the presence of bioactive compounds, which exert a positive effect on human health. In the case of milk, the known bioactive proteins and peptides are mainly derived from the casein fraction, and only a very few from the soluble protein (whey) fraction. The proteins associated with the milk fat globule membrane (MFGMP), representing 2-8% of the total protein milk content, derive from the apical membrane of secreting epithelial cells of the mammary gland (MFGM). While MFGMP have a very low classical nutritional value, they play important roles in various cell processes and defence mechanisms for the newborn. Up to now only a very few MFGMP have been identified and characterized in terms of their biological activity.
Among the immune factors, secretory immunoglobulin A (sIgA) have recently been shown to be strongly associated with the MFGM and to be found in the stools of the newborn, suggesting an additional protective mechanism throughout the intestine. Recently have been recognized also non immunological components of human milk fat globules, namely mucin, lactadherin, glycoaminoglycans and oligosaccharides, which can also protect breast-fed infants against infection by micro-organisms. Among the limiting factors for membrane proteins analysis by 2-DE, the most difficult problems to solve are those linked to the hydrophobicity, which limits protein solubilization. That is probably why only one 2-DE map of MFGMP has been reported to date, where a rather small number of proteins were identified, and only by immunoassay. The aim of this study was to increase the knowledge of human milk protein composition, so as to produce a standard reference as close as possible to reality, the so-called "gold standard", for the production of formula milk. Formula-fed babies, and even babies fed in the very first days of life with human milk from a Lactarium, completely lack the immediate and long term beneficial effects of human colostral MFGMPs. To produce more functionally adeguate formulas or fortified human milks, a further knowledge of human MFGMP composition is required. The practical aspect of this study aimed to update the structural proteome of human colostral MFGMPs and to create an annotated 2-DE MFGMP database available on line. One hundred and seven 2-DE spots derived from human colostral MFGMPs were investigated by MALDI-TOF mass spectrometry, and the proteins were identified by three different software packages available on the web (Peptident, MS-Fit and Profound); the uncertain identifications were solved by nanoESI-ion trap mass spectrometry using Sequest software. An update of the proteins associated with MFGMP and, more remarkable, the availability of this information on-line as an annotated 2-DE map (http://www.ispa.cnr.it ), may be necessary in functional proteomic studies dealing with the physiology and pathology of the mammary gland, and with achieving formulated milks that are more similar to the "gold standard" represented by maternal milk.
Immagini: