The growing interest in metal nanoparticles (NP) is related to their increasing importance for a series of applications, ranging from nanoelectronics to magnetism, pharmaceutics (e.g. for biological labeling) and catalysis. It is now well established that the NPs' properties depend critically on their size and shape1-3 and that some peculiar geometries may appear when reducing the cluster size4. In spite of the vast literature on the topic, most of the attention has concentrated so far on metal NPs made of a few tens of atoms or more2. For metal clusters of just a few atoms supported on insulating surfaces5-8, the catalytic activity is size-dependent and it is directly related to the intrinsic electronic and geometric properties of the NPs and to the size-dependent NP-substrate interaction. Considering the technological relevance of these nano-objects, a clear description of their properties also in the very low size limit is important. This is particularly true in the case of Ni, which combines its magnetic nature to a strong catalytic activity.
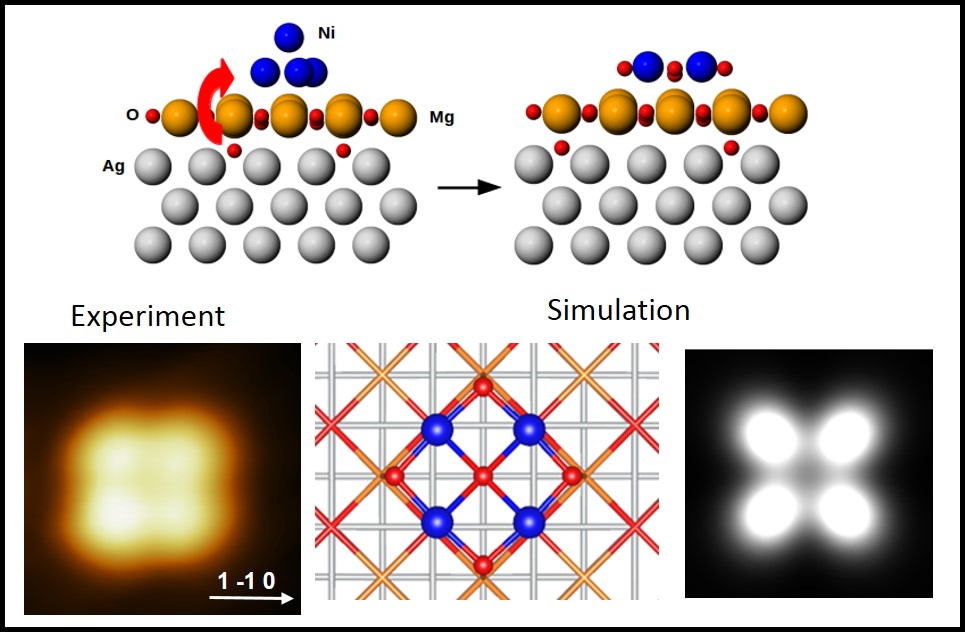
In the present work we have studied Ni nanoclusters grown on MgO/Ag(100) ultrathin films (just one monolayer thick) at T=200 K. MgO is a very common substrate for the deposition of metal NPs, both for its importance in applications and for its simple atomic and electronic structure and wide band gap. In spite of that, the optimal experimental conditions for growing flat and extended monolayer films on Ag(100)9,10 were found only recently. The present work deals with the growth of Ni clusters on such films. We have shown by low temperature scanning tunneling microscopy (LT-STM) analysis and density functional theory (DFT) calculations that, in the limit of low Ni coverage, nanoclusters of four to six atoms form and that these aggregates are flat rather than three-dimensional as expected for pure Ni aggregates. Both the planar structure and the interatomic distance between neighboring Ni atoms are indicative that the nanoparticles do not consist of pure metal atoms but that NiyOx has formed. Excess oxygen atoms are indeed present at the MgO/Ag interface. They reduce the stress in the oxide layer favoring the formation of extended terraces10. Such atoms are pumped by the Ni atoms deposited on the MgO monolayer, which are oxidized to NiyOx.
Besides being of interest in view of the different catalytic properties of Ni11-13 and NiO14,15, our results demonstrate once more the peculiarity of ultrathin oxide films with respect to the corresponding bulk materials and suggest that the physics of these systems is still far from being completely understood.
Financial help from Compagnia S. Paolo and from ICTP (through a post-doctoral grant) is acknowledged.
M. Smerieri, J. Pal, L. Savio, L. Vattuone, R. Ferrando, S. Tosoni, L. Giordano, G. Pacchioni, M. Rocca; J. Phys.Chem. Lett. 2015, 6, 3104; DOI: 10.1021/acs.jpclett.5b01362
(1) Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.-J., Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights Accounts of Chemical Research 2013, 46, 1673.
(2) Sitja, G.; Le Moal, S.; Marsault, M.; Hamm, G.; Leroy, F.; Henry, C. R., Transition from Molecule to Solid State: Reactivity of Supported Metal Clusters Nano Letters 2013, 13, 1977.
(3) Valden, M.; Lai, X.; Goodman, D. W., Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties Science 1998, 281, 1647.
(4) Rossi, G.; Anghinolfi, L.; Ferrando, R.; Nita, F.; Barcaro, G.; Fortunelli, A., Prediction of the structures of free and oxide-supported nanoparticles by means of atomistic approaches: the benchmark case of nickel clusters Physical Chemistry Chemical Physics 2010, 12, 8564.
(5) Heiz, U.; Sanchez, A.; Abbet, S.; Schneider, W. D., Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: Each atom counts Journal of the American Chemical Society 1999, 121, 3214.
(6) Marsault, M.; Sitja, G.; Henry, C. R., Regular arrays of Pd and PdAu clusters on ultrathin alumina films for reactivity studies Physical Chemistry Chemical Physics 2014, 16, 26458.
(7) Sanchez, A.; Abbet, S.; Heiz, U.; Schneider, W. D.; Hakkinen, H.; Barnett, R. N.; Landman, U., When gold is not noble: Nanoscale gold catalysts Journal of Physical Chemistry A 1999, 103, 9573.
(8) Pacchioni, G., Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces Physical Chemistry Chemical Physics 2013, 15, 1737.
(9) Jagriti Pal, M. S., Edvige Celasco, Letizia Savio, Luca Vattuone, Mario Rocca, Morphology of monolayer MgO films on Ag(100): switching from corrugated islands to extended flat terraces Phys. Rev. Lett. 2014, 112, 126102.
(10) Pal, J.; Smerieri, M.; Celasco, E.; Savio, L.; Vattuone, L.; Ferrando, R.; Tosoni, S.; Giordano, L.; Pacchioni, G.; Rocca, M., How Growing Conditions and Interfacial Oxygen Affect the Final Morphology of MgO/Ag(100) Films Journal of Physical Chemistry C 2014, 118, 26091.
(11) Kim, B.-H.; Yang, E.-H.; Moon, D. J.; Kim, S. W., Ni/MgO-MgAl2O4 Catalysts with Bimodal Pore Structure for Steam-CO2-Reforming of Methane Journal of Nanoscience and Nanotechnology 2015, 15, 5959.
(12) Dan, M.; Mihet, M.; Tasnadi-Asztalos, Z.; Imre-Lucaci, A.; Katona, G.; Lazar, M. D., Hydrogen production by ethanol steam reforming on nickel catalysts: Effect of support modification by CeO2 and La2O3 Fuel 2015, 147, 260.
(13) Vesselli, E.; Rizzi, M.; De Rogatis, L.; Ding, X.; Baraldi, A.; Comelli, G.; Savio, L.; Vattuone, L.; Rocca, M.; Fornasiero, P.; Baldereschi, A.; Peressi, M., Hydrogen-Assisted Transformation of CO2 on Nickel: The Role of Formate and Carbon Monoxide Journal of Physical Chemistry Letters 2010, 1, 402.
(14) Hu, J.; Zhu, K.; Chen, L.; Yang, H.; Li, Z.; Suchopar, A.; Richards, R., Preparation and surface activity of single-crystalline NiO(111) nanosheets with hexagonal holes: A semiconductor nanospanner Advanced Materials 2008, 20, 267.
(15) Zion, B. D.; Sibener, S. J., Molecular beam induced changes in adsorption behavior of NO on NiO(111)/Ni(111) Journal of Chemical Physics 2007, 127.
Immagini: