A study by Italian researchers of CNR-IBPM and Sapienza University of Rome published in the Nature Structural and Molecular Biology was able to depict the structural features of a misfolded intermediate. This study reports the first structure of a misfolded state and represents the first step towards our understanding of the misfolding cascade, the cause of several human pathologies.
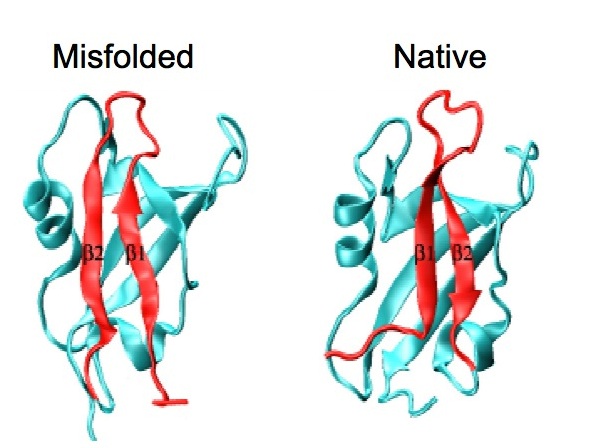
Incorrectly folded states transiently populated during the protein folding process are potentially prone to aggregation and have thus been implicated in a range of misfolding disorders that include Alzheimer's and Parkinson's diseases. Despite their importance, however, the structures of these states have largely escaped detailed characterization because of their fleeting nature.
By studying the folding pathway of a simple model system, such as the PDZ domain family, and by applying a combination of experimental and theoretical approaches, Stefano Gianni, Maurizio Brunori and colleagues, in collaboration with Prof. Michele Vendruscolo from the University of Cambridge (UK), provided the first detailed structural characterization of a misfolded state. Surprisingly, the misfolded intermediate displays an overall topology that is largely native-like, with minor folding defects that are mainly located in the ligand-binding pocket of the protein, suggesting that folding and function might trigger conflicting constrains.
In the context of the fight against many neurodegenerative diseases, this work paves the way to future studies aimed at restoring the function of misfolded proteins by stabilizing their native fold.
Vedi anche:
Immagini: