Conventional batteries are already unable to deliver power in more and more shrunk volumes maintaining the requirements of long duration and light weight. A possible solution to overcome these limits is the use of miniaturised fuel cell. The fuel cell offers a greater gravimetric energy density compared to conventional batteries. They are typically realised using an electrolyte material, usually a polymer membrane (proton exchange type), that is encapsulated between electrodes. Fuel cell operates when fuel (usually hydrogen) is delivered toward one of the two electrode while oxygen flows toward the opposite electrode. By heating the electrode-electrolite structure fuel and oxygen trigger the electrocatalytic reactions with the catalitic material and produce free electrons which may be collected in an external circuit, while positive ions flow through the membrane and form water close the other electrode.
The activity of the research team of the Section of Catania of IMM-CNR allowed to demonstrate that the integration of the complex structure of a micro-fuel-cell on silicon is possible by using only micromachining, fully compatible with the standard ULSI silicon technology.
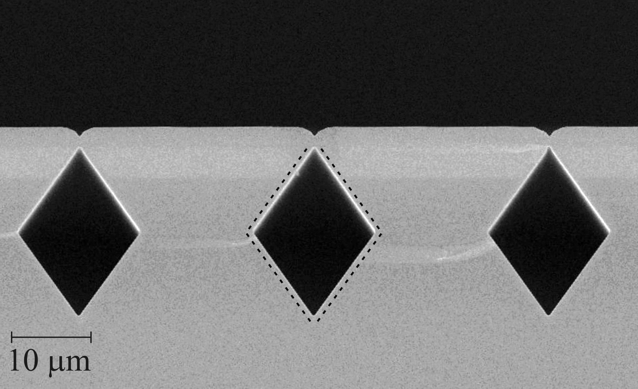
We started by designing an extremely innovative structure depicted in Figure 1.
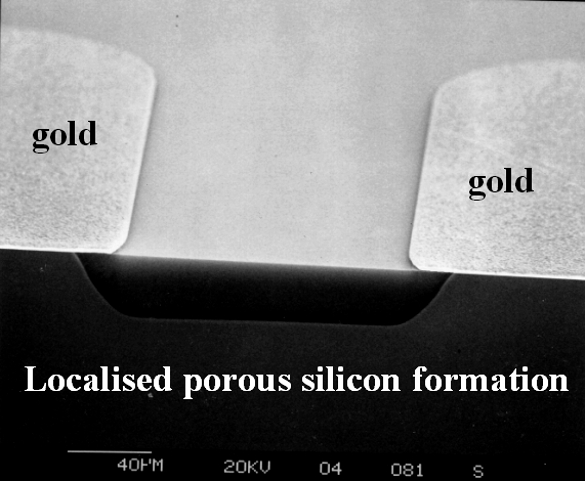
The developed technologies start from the realisation of buried microchannels to deliver fuel and oxygen. This process (protected by a pending patent) is based on the creation deep trenches on the silicon surface followed by anisotropic KOH etch along the different crystallographic planes of crystalline silicon. The created channel is then buried under the surface by silicon epitaxy without any crystallographic defect (Figure 2). The next step consists in the deposition of gold strips between adjacent microchannels by standard photolithographic technique. These gold interconnects will allow us to collect current generated by the fuel cell in an external circuit. The integration of the polymer proton exchange membrane is realised after transforming the silicon region above each microchannel in a porous structure by a proper electrochemical process (Figure 3).
Within this porous structure clusters of catalytic metals (Pt or Ru) are incorporated by electrochemical deposition: they are responsible of the chemical reaction producing electrical free charges. Only after this sequence all the structure is finally impregnated with the polymeric proton exchange membrane which can contact the catalytic clusters. Following this procedure we succeeded to fabricate both half elements of our integrated Si-based micro-fuel-cell. The proposed structure can easily scaled down, it is compatible with all the other standard ULSI processes and, very important, it is well adaptive to many possible architectural developments for a wide range of application in the field of portable electronic devices. Although we are at the beginning of our research activity, the developed enabling technologies give significant advantages to the Catania team and we are confident that in a couple of years the first electronics devices fully or partially powered by integrated micro-fuel-cells will be available.
Vedi anche:
Immagini: