15/05/2023
The Human Genome Project—the complete sequencing of an individual genome—was a monumental milestone in modern biology. Yet, to uncover what underlies our individuality, along with our similarities, a comprehensive understanding of human genetics requires a comparative approach based on many diverse complete genomes.
In new research published in Nature on May 11, 2023, the Human Pangenome Reference Consortium presents the assembly of 94 human genomes to investigate genetic variation between humans. By incorporating information from many diverse individuals, the draft human pangenome reference provides a more equitable foundation for biomedical research. Authors show that by providing a reference that is more representative of all of us, the draft human pangenome can improve the accuracy of genotyping, and that it supports the discovery of almost twice as many structural variants as is possible with a single reference genome.
Leveraging long read sequencing and new bioinformatic techniques that support the comparison of many complete human genomes, it was possible to decipher the sequence variation of genomic regions that were previously inaccessible, revealing unforeseen patterns of genetic evolution across chromosomes. In a companion paper published in the same issue of Nature, HPRC collaborators used this resource to answer long standing questions about the most common kind of chromosomal abnormality in humans. The unexpected findings serve as an example of how curiosity and collaboration can lead to significant insights for human health.
Insight into chromosome translocations
For the first time, analysis of assemblies from the Human Pangenome Reference Consortium reveals how specific translocations—a piece of one chromosome breaks off and attaches or fuses to another—called Robertsonian translocations, can form. Robertsonian translocations are the most common type of chromosomal fusion in the human population, occurring in 1 out of every 1,000 individuals, and contribute to infertility and genomic abnormalities like those that cause Down syndrome. The molecular basis for this type of translocation had until now remained elusive.
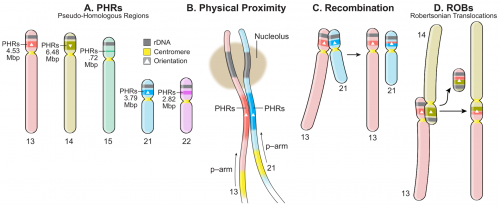
Humans have 23 pairs of chromosomes—the DNA/protein complex with two arms extending from the centromere, a region approximately at the center of the arms where duplicated chromosomes are held together prior to cell division. Five of the pairs do not look like the others. Termed human acrocentric chromosomes, these have asymmetric arm lengths, with one short arm and one long arm, compared with the remaining chromosomes. Of particular biological significance, the short arms of acrocentric chromosomes contain the genes required for the synthesis of ribosomes and ribosomal RNA. Ribosomes are required for manufacturing proteins, the workforce of a cell, and their formation is a cell’s most energy intensive activity.
During meiosis, a type of cell division that gives rise to sperm and eggs, genetic material can be exchanged or swapped between two paired chromosomes. This process, called recombination, where pairs of homologous chromosomes—one paternal and one maternal—break, and segments are either exchanged or copied, increases genetic diversity in offspring because the chromosomes at the end of meiosis differ both from the parent and from each other.
An unexpected recombination result
However, analysis performed by the leader of the collaboration, Erik Garrison, Ph.D., of the University of Tennessee Health Science Center (UTHSC), revealed evidence for a different type of DNA exchange. Specific regions along the short arms of chromosomes 13, 14, 15, 21, and 22 are remarkably similar, indicating that recombination is occurring between chromosomes of mismatched pairs (for example, chromosomes 13 and 14 are exchanging information). The authors termed these “pseudohomologous” regions, to indicate that although they occur on different chromosomes, during meiotic recombination, they exchange sequences with other members of the acrocentric chromosome community as if they were homologs, or true pairs.
These findings were achieved through the application of a novel bioinformatic approach. The team built a pangenome graph, which essentially acts as an alignment, or framework, to represent all possible similarities in sequence from dozens of genomes. Analyzing the graph’s structures enabled the researchers to infer the presence of regions on different chromosomes that are being homogenized, or made similar, via recombination.
“We knew that there were regions of homology between the five acrocentrics, but we were amazed by the clarity and consistency of signals of recombination between heterologous acrocentric chromosomes in the pangenome,” said Garrison. “It was a eureka moment when we realized the same regions are involved in forming Robertsonian translocations,” added Gerton. “These regions are clearly undergoing frequent recombination—Robertsonian translocations represent a mistake in the process.”
“Embracing a population-based approach with the HPRC draft human pangenome, we've shed new light on genome evolution and recombination mechanisms in the acrocentric chromosomes”, said Andrea Guarracino, Ph.D. (https://andreaguarracino.github.io/), a Postdoctoral Fellow at UTHSC and the study’s first author. “Our work addresses limitations of previous studies and lays a strong foundation for future genomic and cytogenetic research, moving us closer to unlocking the mysteries of human genetic evolution.”
Answering a 50-year-old mystery
Robertsonian translocations, first observed 50 years ago, result in the formation of a single chromosome when the long arms of two different acrocentric chromosomes fuse near their centromeres. The two short arms are lost amid repeated cell divisions and typically do not cause problems for their human hosts.
However, individuals with this type of translocation are at greater risk for infertility and their offspring are more prone to trisomy conditions (having three copies of a chromosome instead of the usual pair) like Down syndrome, also known as Trisomy 21 (three copies of chromosome 21).
“Lots of people are Robertsonian carriers and don’t know it. Essentially you have a chromosome with an extra piece and during reproduction, this piece plus two normal chromosomes is similar to a trisomy,” said Gerton. “Inheriting the incorrect number of chromosomes, like Trisomy 21, increases with maternal age, however Robertsonian translocations are the most common cause of non-age associated Down syndrome.”
On chromosome 14, an inversion—a type of rearrangement where a chromosome segment breaks and rejoins in the opposite direction—in the pseudohomologous region relative to the other acrocentrics is strongly suspected as the culprit for Robertsonian translocation between chromosome 13 and 14, and 14 and 21, an idea the group plans to test in the future. Gerton explained, “This may ultimately lead to a better mechanistic understanding of what causes Down syndrome and other genetic disorders.”
The collaboration also paves the way toward considering these regions in biomedical and evolutionary studies that have previously overlooked them. “We haven’t been able to account for variation in the short arms of the acrocentrics in past studies of genome wide association and human evolution,” said Vincenza Colonna, Ph.D., a collaborator at UTHSC and the Institute of Genetics and Biophysics at the National Research Council of Italy. “We show that these regions behave unusually genetically, and new approaches will be needed to leverage the information they contain into biomedical studies at the population level.”
CNR co-authors include Vincenza Colonna, Ph.D., Silvia Buonaiuto, Ph.D.
Additional authors:
- at University of Tennessee Health and Science Center include Prof. Erik, Garrison, Ph.D., Andrea Guarracino, Ph.D., Flavia Villani, graduate student
- the Human Pangenome Reference Consortium.
This work was supported by Consiglio Nazionale delle Ricerche, Italy, the Tennessee Governor’s Chairs program.
Per informazioni:
Vincenza Colonna
Cnr - Igb
vincenza.colonna@igb.cnr.it
Vedi anche:
